Becton Dickinson – Sustainable Product Opportunity
Becton Dickinson (BD) is one of our largest suppliers across multiple framework agreements, particularly in the consumables space, both in terms of value and volume.
Over the past year, we’ve been strengthening our relationships not only with BD’s category teams but also with their ESG (Environmental, Social, and Governance) lead.
This collaboration is opening up exciting opportunities across products, clinical pathways, and packaging. Given the scale of BD’s portfolio, our initial focus has been on identifying where the most impactful sustainability opportunities lie.
We’ve already uncovered several promising examples, and over the next 12 months, we aim to work closely with BD, our category teams, and customers to highlight, verify, and quantify these sustainability benefits.
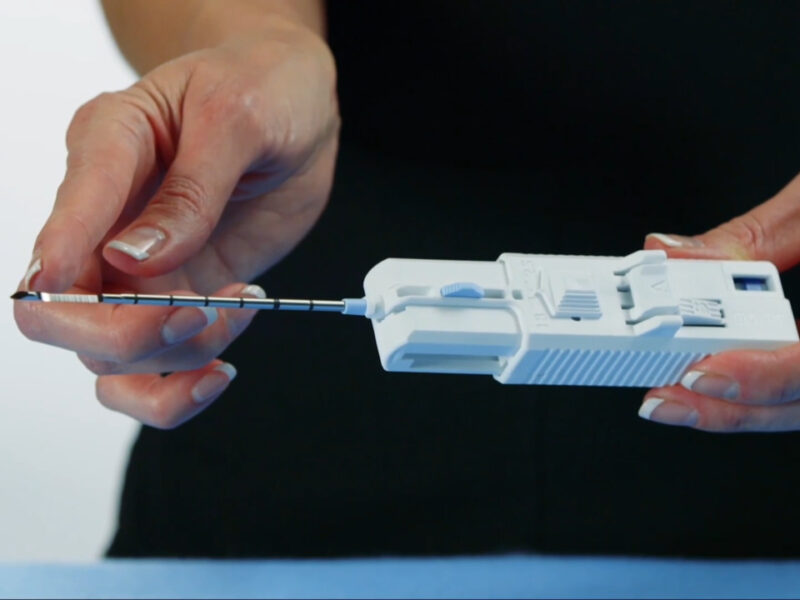
A Case in Point: The Marquee Disposable Core Biopsy Instrument
One standout example is the Marquee Disposable Core Biopsy Instrument, designed for soft tissue biopsies (for example liver, kidney, prostate, spleen, lymph nodes, and soft tissue tumours). From a sustainability perspective, this device enables a ‘TP (Transperineal) full freehand’ technique that eliminates the need for a single-use plastic needle guide, traditionally disposed of as clinical waste.
This change has been well received by clinicians aiming to reduce single-use plastics and streamline the products used in procedures. The intervention is relatively simple to implement in operating theatres and departments.
The NHS Supply Chain Marquee Kit product codes are:
- MQK1820
- MQK1825.
Please contact BD about this approach as there is clinical support and training available for implementation. See our Useful Links section.
Comparing Techniques
| Component | Old TP Technique | New TP Technique (Marquee) |
| Biopsy Needle | Y | Y |
| Ultrasound Probe | Y | Y |
| Needle Guide | Y | N |
By removing the needle guide, plastic waste is reduced and the high costs and carbon footprint associated with clinical waste disposal can be avoided.
Measuring the Impact
While the sustainability benefits are clear, we are now working to quantify them in terms of CO₂ emissions and waste reduction. Four hospitals are currently auditing the TP full freehand technique to assess both patient and clinician experience. Additional analysis is underway to evaluate sample quality and diagnostic yield, (the comprehensive measure of the performance of a test considering access, specimen availability, sensitivity, and test completion).
Beyond environmental gains, there are potential financial benefits. Eliminating a product from the pathway reduces procurement costs and waste disposal expenses. The improved sample quality may also reduce the need for repeat procedures, enhancing pathway efficiency and potentially lowering the risk of surgical site infections.
We’re committed to working with BD to fully quantify these benefits in the coming financial year.
Broader Opportunities Ahead
This is just one example. Across BD’s extensive portfolio and our framework agreements, there are numerous opportunities to improve sustainability, whether through product innovation, pathway redesign, or packaging improvements.
A key focus has been connecting with Sustainability Managers within our major suppliers and aligning their initiatives with our Category teams. This has given us a broader view of the impactful work already underway, from simple interventions to transformative innovations that could significantly improve early detection, diagnosis, and treatment.
One particularly exciting area is the shift of care from hospitals to community settings, a priority in the NHS 10 Year Plan. This transition could enable procedures traditionally performed in acute settings to be delivered closer to patients, offering both environmental and social sustainability benefits.
Our team is actively exploring these transformational opportunities and working to understand their full value, including their sustainable value.
Links section
-
Syringes, Needles and Associated Products
The framework agreement for the supply of Syringes, Needles and Associated Products.
-
Medical and Surgical Consumables
The Category information page for Medical and Surgical Consumables.
-
Supplier Collaboration
A series of articles highlighting our efforts to work with key suppliers through the Supplier Relationship Management (SRM) programme.
-
Becton Dickinson Marquee™ Disposable Core Biopsy Instrument Kit
View more information about the kit and apply for training on BD's website.
