Pee in Pot (PiP) – A Sustainable Shift in Urine Testing Now Available via NHS Supply Chain
Developed within Somerset NHS Foundation Trust, the PiP (Pee in a Pot) is a sustainable, single-solution pulp vessel that simplifies mid-stream urine (MSU) collection while cutting carbon emissions by up to 85%. Now available through the NHS Supply Chain catalogue, PiP replaces multiple plastic items used in conventional testing, reducing waste, spillage risk, and costs.
Backed by clinical evidence and a multi-year innovation journey, PiP is a powerful example of how NHS-led innovation can drive greener, smarter patient care.
We have been using PiPs in our Day Surgery Centre for a while now and find them sturdy and easy to use. They are sustainable and offer a much greener alternative to plastic, reducing waste without compromising on practicality. A great step forward for both patient care and the environment!
Lisa Strawbridge, Deputy Clinical Manager, Somerset NHS Foundation Trust
Overview
A team based within Somerset NHS Foundation Trust noticed that mid-stream urine testing practices were very variable across different clinical areas, and that testing typically uses many items of sterile plastic. These are then disposed of by incineration.
For such a simple test, it seemed unusual to see such non-standardised and wasteful practices. Upon consulting nursing staff and healthcare assistants, it became clear that there was a real need to develop a better solution with a lower risk of urine spillage.
When further investigating variation in practices locally, it was noted that one clinical area performing a high volume of urine testing used clean pulp to collect urine samples instead of sterile plastic. The team analysed the urine microbiology results for this clinical service and found that the results did not differ when compared with similar services using sterile plastic to collect urine.
With the dual aims of reducing the environmental impact of urine testing and developing a urine testing pathway that was simple and standardised, the team set about designing a solution.
New process for collecting mid-stream urine (MSU) sample
With fewer and more simplified steps for the collection and testing of patients’ urine, there is only one single-use plastic product used.
- Collect in PiP
- Pour into sample tube
- Send to laboratory for testing
Point of care – PiP white pulp blend

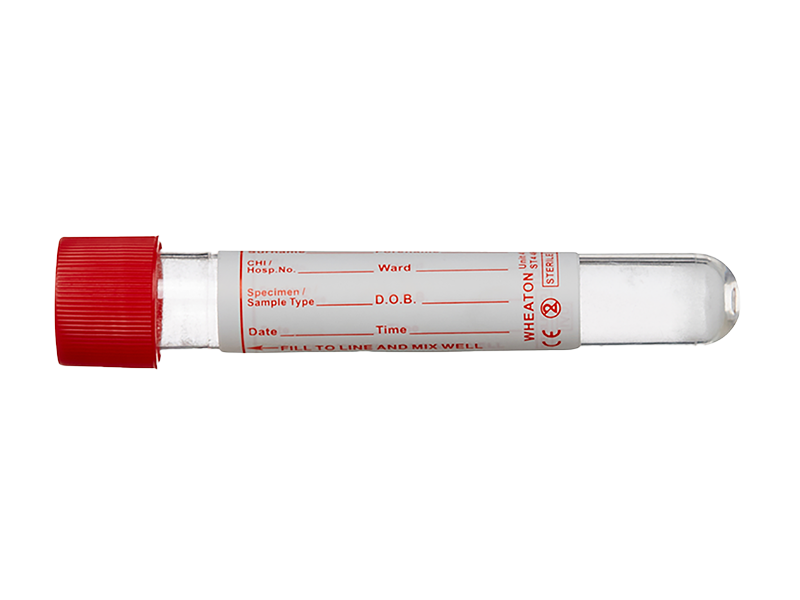
Point of care – 10ml boric acid tube
Pathology laboratory

Urinalysis and reporting
An illustrative example of a previous process for collecting MSU samples
Various processes exist, but in this illustration there are multiple steps and more time consumed:
- Five single-use plastic products used
- Samples handled multiple times.
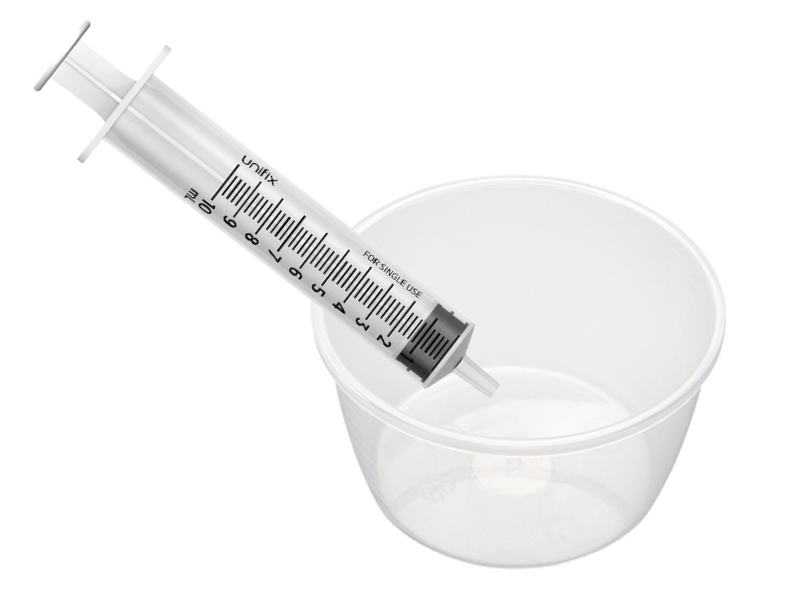
Point of care – 10ml syringe and sterile bowl
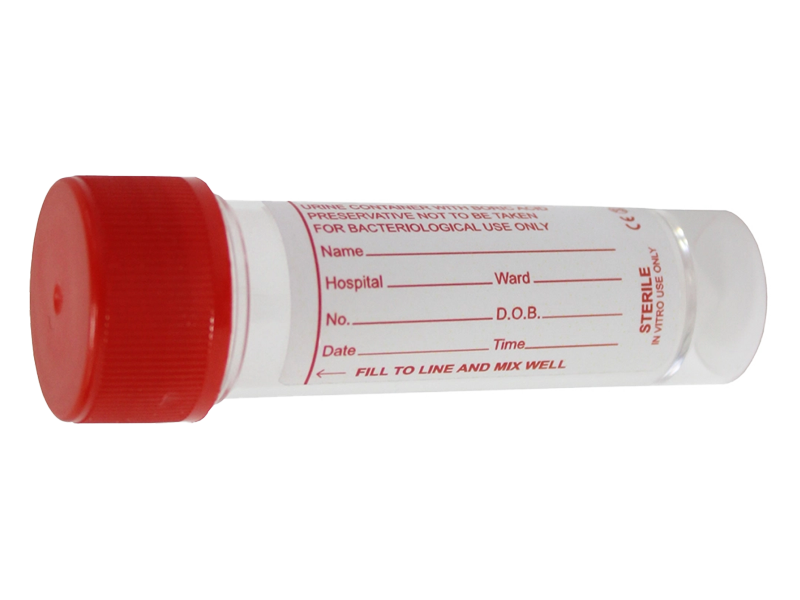
Point of care – 30ml boric acid tube
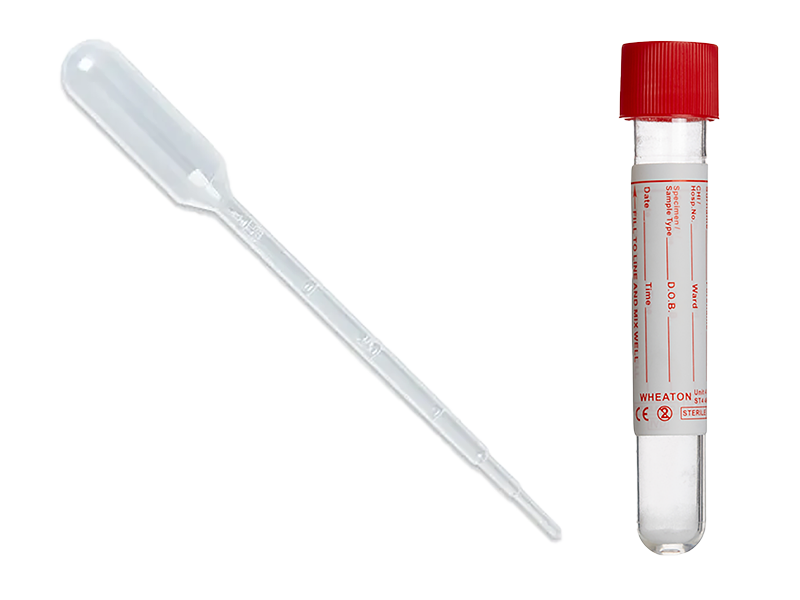
Pathology laboratory – sample transfer using pipette
Pathology laboratory

Urinalysis and reporting
The challenge
The ambitious challenge was to create a single-solution product that allows simple urine collection, bedside testing, and decanting of an appropriate sample volume into a boric acid tube for onward testing in the laboratory.
This product would replace the multiple items commonly used to collect and transfer urine, which in the simplest scenario still involved a sterile plastic bowl and a syringe.
The additional challenge was to create this innovative product out of a more environmentally sustainable material than sterile plastic, such as organically derived pulp. With this in mind, the product must also be able to hold urine whilst maintaining its shape over a prolonged period, and provide non-inferior microbiological performance compared to sterile plastic.
The solution
The PiP (Pee-in-Pot) was developed and iteratively improved over five years of user testing and product development. The PiP urine collection vessel allows collection of urine by the patient and bedside dipstick testing.
After this, the shape of the PiP means that excess urine can be decanted to leave exactly 10ml of urine in the vessel. This is the exact amount needed to fill a 10ml boric acid tube, which can then be filled with the urine and sent to the laboratory for onward testing.
The PiP contains an innovative funnel-shaped end, which is designed to snugly fit within and hold the 10ml boric acid tube. This allows for the urine to be transferred from the PiP without any spillage or the need for additional items, such as syringes, to aid decanting.
Process undertaken
Key steps and milestones during this multi-year quality improvement innovation include:
- Identifying the area where practices could be improved and mapping out practice variation through local auditing and consultation.
- Finding out about practices regionally and nationally in primary and secondary care.
- Finding and analysing data to support a potential move away from sterile plastic towards clean pulp. This included local urine testing data and a microbiological assessment of clean pulp for use in the operating theatre setting performed by the Southwest Academic Health Sciences Network in 2015.
- Bringing together a multi-disciplinary team to design a novel solution to the problem.
- Gaining support from key stakeholders within the trust, including the trust innovation team and senior executives, microbiology clinicians and laboratory teams, infection prevention and control, ward nursing teams, and the trust improvement team.
- Liaison with a manufacturer to produce product prototypes which were iteratively improved.
- Several rounds of local testing in a small number of wards, which included monitoring microbiology performance and user feedback to further inform product design and user instructions. Instructions included how to store and handle the PiP.
- Product registration with the MHRA.
- Product design rights registration.
- Prospective design of a definitive microbiological study to compare PiP with standard of care urine collection. This involved writing a study protocol and consultation with microbiology and statistics experts to inform the study design.
- Successful application for Small Business and Research Innovation (SBRI) funding, resulting in much needed financial support to support the conduct of the study and product commercialisation. The vital funds allowed a healthcare assistant to be employed to successfully run the study.
- Further engagement and agreements with a manufacturer to plan product scale up and packaging.
- Engagement with NHS supply chain after conclusion of the study, which identified the PiP to be non-inferior to the standard of care technique for collecting mid-stream urine specimens for microbiological testing.
The successful development of the PiP to the point of being ready for commercialisation demonstrates the value of being able to innovate within the NHS, and what can be achieved with NHS support and by involving a multi-disciplinary team.
The results
A cradle to grave carbon foot printing was performed to compare the PiP with a range of different common urine testing scenarios.
Greenhouse Gas Protocol Product Standard guidance was applied, and a range of different scenarios were modelled. In all modelled scenarios, testing with the PiP produced lower greenhouse gas (GHG) emissions.
For the base-case, the estimated carbon footprint of PiP testing was 95g CO2e, compared with 270g CO2e for current standard of care testing involving a plastic bowl and a syringe. This is a 65% reduction in GHG emissions.
The emissions reduction was realised for several key reasons:
- The PiP is a single item solution for urine collection, bedside testing, and decanting.
- It is made from pulp instead of plastic, which has a lower manufacturing carbon footprint.
- It can be disposed of using a macerator instead of being incinerated.
Cost savings
Based upon a calculation of direct savings which we forecast across Somerset NHS Foundation Trust and a forecast minimum MSU test volume of 21,119 a year during 2025, we estimated that for us as a provider with 1,020 in-patient beds we would make a direct saving on incineration costs of (plastic) with a value of £264 per annum based upon our incineration costs per tonne of plastic waste. This is probably an under-estimation of the total savings as the total number of urine collections exceeds the number reported by the laboratory as only around 50% of MSU samples are sent for culture.
We also calculated that the savings on consumable plastic items are primarily miscellaneous urine collection vessels, noting considerable variation in practice between clinical services within our trust as an initial urine catch sample technique. Some use a grey pulp product, but we would not discontinue that practice. Items which have been switched out from our ordering system are the 30ml boric acid tube, single use plastic vessels (sterile) typically a 500ml vessel, syringes and pipettes used for urine decant. As a conservative estimate we have saved £6,900 per annum in consumable costs. That will vary between providers of healthcare.
Next steps
In February 2025 the PiP product (NPC FBF85178) was added to the NHS Supply Chain Medical Pulp framework, and is available for customers to order through the catalogue.
See our Useful Links section for more information on this product.
The PiP product is currently available as an eDirect product line with a minimum order quantity of 250. NHS Supply Chain endeavour to offer this product as a stocked line which would lower the minimum order quantities for trusts however this requires a wider uptake across the NHS. We envisage that by promoting the PiP product across the NHS and the benefits to switching to the PiP that NHS Supply Chain will be able to offer the product via direct routes as soon as possible.
See our Useful Links section to learn more about the PiP product and the team who developed it.
NHS Supply Chain and the PiP team are exploring a future webinar to shine a spotlight on this home-grown innovation, watch this space!
See our Useful Links section for information about our Strategy Opportunities and Innovation program.
A detailed comparison has been undertaken of conventional (lean) urine collection processes and compared to the PiP by Mr Joseph John- Urology Clinical Fellow and GIRFT Fellow (Sustainability), that has demonstrated that the PiP produces net carbon savings of between 77-85% across the MSU collection pathway.
Quotes
The introduction of the PiP across Somerset NHS FT in a range of care settings has revolutionised the way in which we approach mid-stream urine collection, it has significantly reduced the carbon footprint associated with what was a highly variable and labour intensive process; it has saved on plastic waste incineration costs and a number of disposable plastic items that we have swapped out for the PiP and significantly saved healthcare assistant time which has been re-directed to frontline patient care activities. The laboratory has also saved laboratory assistant time in a simplified process by not having to decant urine into a urinalysis tube, as the boric acid tube we have chosen fits nicely into our urine analyser
Nael Clarke, Chair of the Somerset NHS FT Innovation Forum and Member of the Somerset NHS FT Strategic Sustainability Forum
Current Urine Collection is non-standardised and relies on using sterile single-use plastics. In use it has gained positive patient and staff feedback. We have also shown that clean pulp (thermofibre) has not resulted in increased rates of contamination
Mr Nick Burns-Cox, Consultant Urologist and Primary Clinical Innovator and Member of the Somerset NHS FT Green Care Action Group
The PiP is a 100% biobased solution, designed for collecting urine specimens, which is manufactured from a sustainable material called bagasse, which is a recyclate from sugar production, which is converted into a virgin fibre from which the PiP is produced from. The PiP has been developed as a more streamlined and sustainable alternative to the current method of collecting urine specimens for analysis, which typically involves several single use plastic items, and several stages to the process. The PiP is manufactured from a renewable material and can be disposed via a macerater, resulting in a considerably more sustainable product from the point of manufacture to the point of disposal, compared to products currently in use
David Cardall, Head of NHS and Healthcare Sales, Polyco Healthline Ltd
Links section
-
Urine Sampling Cup
NHS Supply Chain Online Catalogue - Pee in Pot 350ml (NPC FBF85178).
-
Pee in Pot (PiP) Product Team Video
To learn more about the PiP product and the team who developed it please view this video on YouTube.
-
Strategy Opportunities (Innovation)
An overview of all of the available Medical Technology Innovation Opportunities and adoption guidance.
