New Innovative Kurin Lock® Blood Collection Device Improves Efficiencies in Clinical and Laboratory Teams
Background
NHS Supply Chain work with partners such as NHS England, NHS Improvement, the National Institute for Health, Care Excellence (NICE), and the Academic Health Science Network (AHSN) to support the introduction of new technology and innovation into the NHS that provides demonstrable benefits for our patients and users.
To facilitate this, we are utilising a new quicker procurement process called a Voluntary Ex Ante Transparency notice (VEAT) which enables us to be more responsive in bringing innovative products to the market.
The latest innovation to be launched through the NHS Supply Chain route is the Kurin Lock® Blood Collection Device which will be available to order via our catalogue from 29 August 2023.
About Kurin Lock®
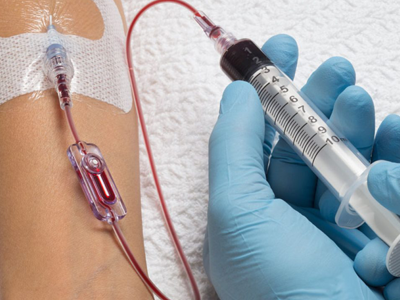
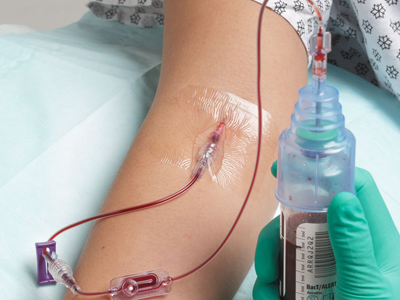
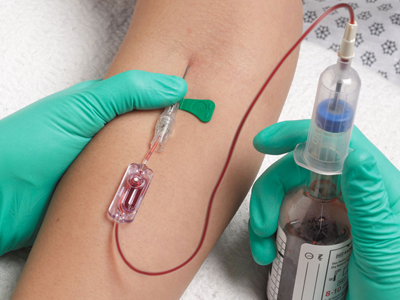
Kurin Lock® from Iskus Health UK Ltd is a blood collection device. The technology is self‑contained and consists of:
- a blood collection tube and needle
- a flash chamber that collects, isolates and shows the first 0.15 ml of blood (flash)
- a blood collection tube that collects the remaining sample to be sent for culturing and analysis.
The integrated Kurin Lock® enables clinicians to side-line the initial flash of blood, which may contain skin microbes, with no change in collection practice. Blood cultures tests are used to detect microorganisms in the blood that lead to the diagnosis of serious infections.
Why is it Innovative?
The Kurin Lock® is recognised as being innovative as it isolates the first flash of blood from the sample which may contain contaminants (for example microorganisms from the skin surface). These could cause false-positive results during blood culture. The device does not allow backflow of blood, and does not add any extra time or work for the person collecting the blood.
What are the Benefits?
Kurin Lock® reduces the number of false positive blood culture test results, which in turn:
- Directly improves efficiencies within clinical and laboratory teams, saving time and money retesting
- Reduces administration of unnecessary antibiotics
- Decreases number of bed days
- Leads to an improved patient experience.
Studies show that best practice compliance combined with Flash Technology to side-line the initial flash of blood can reduce contaminated cultures by more than 80%, improving the clinical value of blood culture testing for safer, more effective, and lower cost treatment of patients.*
*Statistics sourced from https://www.kurin.com/
Customer Insight
A number of trusts have trialled the Kurin Lock® Blood Collection Device and are in the process of seeking business case sign off to enable this to be implemented.
The Clinical and Laboratory Standards Institute recommends that hospital achieve a contamination rate of < 3%. However, the evidence base demonstrates blood culture contamination rates range from 2% – 10% with the highest rates in A & E. Contaminated blood cultures are estimated to cost an additional £2,000 – £4,000 to overall costs of treatment. Despite introduction of regular refresher training, bespoke blood culture packs, guidance against taking blood cultures from newly inserted peripheral cannulae and the introduction of a manual 2ml discard prior to inoculating blood culture bottles the blood culture contamination rates at GSTT have been consistently above 6%. A 6 month trial of Kurin was done in A & E in 2021. The results of this trial showed a 66% reduction of blood culture contamination rates down from 6% to 2%. These successful rates has meant that Kurin has been implemented across the trust for peripheral blood cultures.
Jane Hodson
Lead Nurse VAST and OPAT (Deputy HoN), Infection Prevention and Control
Additional Information
- You can find out more detail about how to order and additional support on our contract information page.
- The Strategy Opportunities for Medical Technology Products document is available to view on our website, this gives an overview of all the available Medical Technology Innovation Opportunities that are available via NHS Supply Chain and any relevant adoption guidance.
- The National Innovation Front Door has identified that this product has innovative technology and is currently in progress to have NICE guidance published.
Links section
-
Blood Culture Collection Systems
The framework agreement for the supply of Kurin Lock Blood Culture Collection Systems.
-
Strategy Opportunities for Medical Technology Products
An overview of all of the available Medical Technology Innovation Opportunities and Adoption guidance, all in one place.
-
Kurin Lock for blood culture collection on NICE website
Evidence-based recommendations on Kurin Lock for blood culture collection.
