Latest News: The first new technology selected for support in 2024 / 2025 is from AposHealth® UK.
AposHealth® UK is an innovative footwear product recommended as a cost-saving treatment for patients unsuitable for knee replacement surgery.
It is available via NHS Supply Chain and supported by the MedTech Funding Mandate policy.
Future NHS Collaboration Platform
A new NHS England MedTech Funding Mandate webinar is now available discussing the key product and patient benefits of AposHealth® UK. To access the recording and supporting slides, please register via the Future NHS Collaboration Platform.
See our Useful Links section to find out more, including a link to the Future NHS, Health Innovation Network (HIN) and NHS Long Term Plan websites, and NICE Guidance MTG76 covering this particular innovation.


About the MedTech Funding Mandate
The NHS Long Term Plan committed to accelerating the uptake of selected innovative medical devices, diagnostics and digital products, supporting commissioners and healthcare providers to bring life-changing innovations to patients quicker, by developing the MedTech Funding Mandate (MTFM) policy. NHS Supply Chain was selected as the preferred supply route.
The proposal for this policy was consulted on in December 2019 by NHS England, with patient representatives, NHS providers, commissioners, charities, academics, delivery partners, consultants, independent health providers and industry. The MTFM policy received strong support, with clear recommendations on the criteria for inclusion technologies.
In April 2021 the MTFM policy was launched to support adoption of MedTech and diagnostic technologies across almost 200 healthcare services. These are reviewed annually to ensure that they still meet the policy criteria, set out in paragraph 17, supported by the published NICE guidance.
To be supported by the MTFM, technologies must meet the policy’s three criteria and align with broader NHS England programmes (for example, Net Zero). These criteria require that technologies:
- Are effective: demonstrated through positive NICE Medical Technology Guidance (MTG) or Diagnostic Guidance (DG).
- Are cost-saving within three years: NICE modelling demonstrates a net saving within 3 years of implementing the technology, demonstrated by a published NICE resource impact template.
- Are affordable to the NHS: the budget impact should not exceed £20 million nationally, in any of the first three years.
Unfortunately, NHS Supply Chain is unable to advise on commissioning or funding arrangements. For support regarding the implementation of the MTFM, please contact your local Health Innovation Network (HIN) or NHS England’s Innovation Team via england.medtechfundingmandate@nhs.net.
See our Useful Links section to find the contact details for your regional HIN.
How is NHS Supply Chain involved?
NHS Supply Chain is the preferred supply route for these products. Each of the products can be ordered through our Online Catalogue and Ordering system.
If you do not currently purchase products through our route then you will need to be set up with an account. See our Useful Links section for information on how to create an account.
Should you have any patient safety or complaints related to one of the MTFM products purchased through NHS Supply Chain, please complete our Products Complaints Form.
The technologies supported by the policy this year (2024 / 2025)
AposHealth® UK
AposHealth® UK is a non-invasive device worn on the feet to reduce pain and improve function in patients with knee osteoarthritis.
The device consists of a pair of AposHealth® UK shoes with two curved pods (pertupods) on the heel and forefoot of each shoe. The pertupods are securely attached to tracks on the bottom of the shoe with screws. Positioning of the pertupods is done by trained healthcare professionals and can be aided by gait analysis software or hardware.
See our Useful Links section to view the relevant NICE Guidance (MTG76) – AposHealth® UK is recommended as a cost-saving treatment for patients unsuitable for knee replacement surgery.
NHS providers and commissioners are encouraged to review NICE MTG76 and to contact us, in order to understand the benefits of adopting AposHealth® UK (as well as other other MTFM supported technologies).
The technologies supported by the policy in 2022 / 2023
GreenLight XPS
GreenLight XPS vaporises prostatic tissue with a laser. The laser fibre (consumable product) is passed through a cystoscope to photoselectively vaporise the enlarged prostate tissue, leaving a clear urethral channel. GreenLight XPS can be done as a day case procedure, reduces the risk of complications, and allows a quicker return to normal activity.
In order to purchase the capital equipment that delivers the treatment (via the NHS Supply Chain framework agreement) please contact a member of the team for a unique order reference number.
Medical Devices Team
The unique order reference number should be quoted on the purchase order raised with the supplier and will ensure that the purchase is covered by the terms and conditions of the framework agreement.
This pricing will not be valid without an NHS Supply Order reference number.
- Green Light XPS (MPC) 0010-0210S-G0
Plasma
Plasma is a bipolar electrosurgery system for transurethral resection and haemostasis of the prostate. The system uses electrodes to cut out (resect) prostate tissue and stop any local bleeding afterwards (haemostasis), which avoids the risk of transurethral resection syndrome and reduces the need for blood transfusion. This procedure can be done as a day case.
In order to purchase the capital equipment that delivers the treatment (via the NHS Supply Chain framework agreement) please contact a member of the team for a unique order reference number.
Medical Devices Team
The unique order reference number should be quoted on the purchase order raised with the supplier and will ensure that the purchase is covered by the terms and conditions of the framework agreement.
This pricing will not be valid without an NHS Supply Order reference number.
To order the consumable product, please use our Online Catalogue.
- Plasma System – ESG-410 Electrosurgical Generator (MPC) WA91307
Rezum
Rezum is a minimally invasive procedure that uses water vapour (steam) to treat BPH. The technology delivers targeted, controlled doses of stored thermal energy in water vapour directly to the region of the prostate gland with the obstructive tissue causing lower urinary tract symptoms (LUTS). Rezum effectively alleviates BPH and patients can be treated as outpatients.
UroLift
The UroLift system lifts and holds the enlarged prostate tissue away from the urethra, relieving the compression of this organ. It can be performed under local anaesthesia in an outpatient setting or ambulatory care centre, and the patient can return home the same day without a catheter.
XprESS multi-sinus dilation system
The XprESS multi-sinus dilation system is a sterile, single-use device for treating chronic sinusitis. Dilation of the XprESS balloon remodels the bony sinus outflow tract by displacing adjacent bone and paranasal sinus structures. This has the potential to reduce the tissue lost compared to traditional functional endoscopic sinus surgery (FESS) procedures.
Thopaz+
Thopaz+ is a portable digital chest drain system that provides regulated negative pressure close to the patient’s chest and continuously monitors and records air leak and fluid drainage. The system comprises an inbuilt, regulated suction pump with a digital display, rechargeable battery, tubing that connects to any standard chest drain catheter and a Thopaz+ disposable fluid collection canister. Sensors in the system turn the pump on and off to ensure the pressure level set by the healthcare professional is precisely maintained.

Spectra Optia Apheresis System
The Spectra Optia Apheresis System is an apheresis and cell collection platform for the treatment of sickle cell disease. In a typical exchange procedure, Spectra Optia separates and removes sickle red blood cells from the patient’s blood using continuous flow and centrifugation. These are replaced with healthy red blood cells according to the user-defined software protocol.
This technology is now available from NHS Supply Chain. Please contact:
Renal team
The technologies supported by the policy in 2021 / 2022
GammaCore
GammaCore is a handheld device which provides non-invasive transcutaneous vagus nerve stimulation (nVNS). It is intended for treatment of adults with primary headache, including migraine, cluster headache, and medication overuse headache.
It administers vagus nerve stimulation non-invasively by delivering a mild electrical stimulation through the skin to either the right or the left branches of the vagus nerve in the neck.
GammaCore differs from other vagus nerve stimulators in being applied to the skin of the neck rather than implanted by a surgical procedure.
The device is easy-to-use and patients can carry it with them so that they can use it regularly to prevent cluster headaches or when they feel one coming on.
I was an early adopter of GammaCore… It’s transformed what I do for patients… It’s reached my aim of managing to treat patients without giving them lots of tablets.
Dr Nick Silver, a neurologist from the Walton Centre NHS Foundation Trust

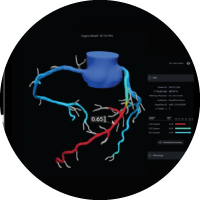
HeartFlow
Coronary heart disease (CHD) is the most common type of heart disease and it is estimated that 2.3 million British people currently live with the disease*. Common symptoms of CHD include chest pain or tightness, shortness of breath and heart palpitations. Some patients may have no symptoms.
The HeartFlow Analysis is an advanced test that uses the latest medical imaging technology and data analysis to show how each blockage impacts blood flow to the heart.
Use of HeartFlow streamlines the diagnostic experience for patients – often helping physicians eliminate invasive diagnostic procedures for those who do not need them. It also helps physicians swiftly and accurately diagnose those who do need invasive procedures. Use of the technology helps physicians reduce redundant non-invasive diagnostic testing, reduces patient time in hospital and face-to-face clinical contact, and helps ensure that hospital visits for those who do need them are streamlined, which is particularly crucial during the COVID-19 pandemic.
The HeartFlow Analysis takes data from a coronary CT angiography (CTA) scan and uses deep learning technology and highly trained analysts to create a personalised, digital 3D model of the patient’s coronary arteries. Its algorithms solve millions of equations to simulate blood flow in a patient’s arteries to help clinicians assess the functional impact of any blockages.
If you have any questions about HeartFlow please contact:
*British Heart Foundation. (2018). CVD Statistics – BHF UK Factsheet https://www.bhf.org.uk.
We know that when we’re using this test [at our site] that around about one in four circumstances where we would have sent the patient for an invasive test, we have managed to avoid that.
Dr Tim Fairbairn, Consultant Cardiologist at Liverpool Heart and Chest Hospital NHS Foundation Trust
Placental growth factor based testing
Placental growth factor (PlGF) tests have been developed to help clinicians and midwives diagnose suspected pre-eclampsia (PE) in pregnant women and to assess the risk for complications associated with PE.
Quidel and Roche Diagnostics are the two suppliers selected under the MTFM to provide these tests.
Roche Diagnostics – Elecsys sFlt-1/PlGF ratio test: The Elecsys ratio test measures two circulating placentally derived biomarkers; soluble FMS like Tyrosine kinase 1 (sFlt-1) and Placental Growth Factor (PlGF). These biomarkers are detectable in the circulation of pregnant women and their levels are altered in PE; sFlt-1 becomes elevated and PlGF is decreased.
With the Elecsys test, the levels of sFlt-1 and PlGF are measured then converted into a ratio which can predict the women who will not get PE in the following seven days. The Elecsys test can be run on any Roche Elecsys or Cobas e automated analyser in a lab environment.
Quidel Corporation – Quidel Triage PlGF test: The Quidel Triage PlGF test is a quantitative immunoassay for the measurement of PlGF. Circulating maternal PlGF levels are abnormally low in pregnancies with defective placentation and the test is used to help detect abnormal placentation in pregnancies where there is clinical suspicion of preterm PE.
The Quidel Triage PlGF test is run on the Triage MetroPro analyser. It is positioned as either a point-of-care or lab-based test.
In my first two pregnancies I [was] constantly in and out of hospital as I had high blood pressure and protein in my urine, and, with my first, I was eventually diagnosed with pre-eclampsia. During my recent pregnancy I had some of the symptoms associated with pre-eclampsia again – high blood pressure, headaches, protein in the urine – but this time I had a simple and quick blood test. The test showed that I didn’t need to be admitted to hospital and I was able to go home and be with my family, with the peace of mind that I was okay.
Rebecca Sanderson, a patient who benefited from PlGF based testing
To order the consumable products, please use our Online Catalogue.


SecurAcath
SecurAcath is a single-use device to secure percutaneous catheters in position on the skin.
It is intended for use in adults and children who need a central venous catheter (CVC). This is a long, thin, flexible tube that is inserted into a vein through the skin.
It is positioned so that the distal tip lies in a large central vein, usually the superior vena cava, right atrium or inferior vena cava.
The benefits of using SecurAcath are:
- One SecurAcath secures for the life of the line
- Dramatically reduces catheter dislodgement and migration
- Catheter remains secure during dressing changes
- Decreases catheter replacement costs
- Reduces catheter related infections and other complications
- Prevents therapy interruption
- Allows easy catheter repositioning if catheter tip must be pulled back
- Improves vessel health and preservation
- Lowers total costs of patient care.
The cost savings are quite noticeable and come from reducing the need to replace lines and no longer needing to double-dress the area. The reliability and the impact this has on patient care is the most important factor, which is why I’m such an advocate.
Carol McCormick, Clinical Interventions Lead Nurse, The Clatterbridge Cancer Centre NHS Foundation Trust