Supplier Newsletter – June 2024
Welcome to our new look supplier newsletter, bringing together our latest news and updates in an easy-to-read format.
Please contact us if you would like to:
- Engage with the Supplier Relationship Management (SRM) team to learn more about the changes.
- Provide feedback on this new layout and any topics you would like us to include in future editions.
- Be added to our newsletter subscribers list.
Supplier Relationship Management (SRM) Team
Enhancing our Supply Chain to Help Put Patients First
Globally, supply chains will continue to face challenges in 2024, from a shortage in raw materials, to disruptions in manufacturing and transport.
In response, we are building organisational flexibility, capabilities and resilience into our supply chain, whilst limiting our environmental impact.

International Recognition of Medical Devices
Initiative from MHRA signals an important step towards a new regulatory framework for medical devices in Great Britain.

Introducing Our Newly Appointed Commercial Director
Richard Evans shares his plans on leading the continued transformation of commercial capabilities at NHS Supply Chain.

Gorsey Point Customer Migration Nearing Completion
Gorsey Point, our new Regional Distribution Centre (RDC) for the North West, is on track to be fully operational in July 2024.
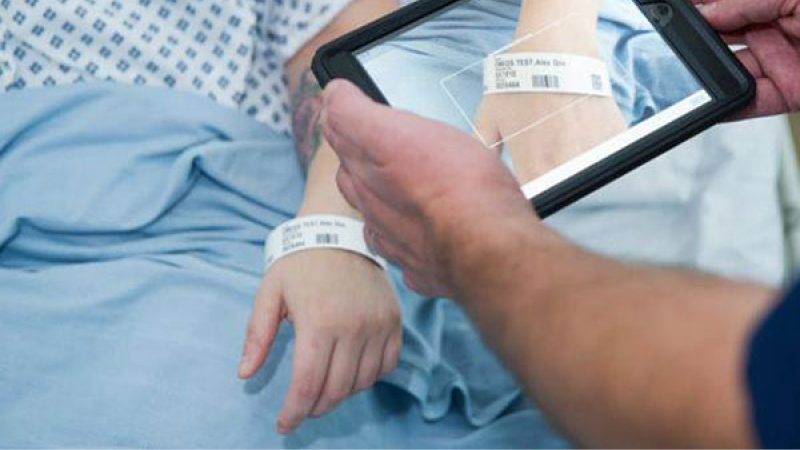
New Data Standards Policy: Supplier Product Coding
Key supplier policy and guidance update – improving patient safety through better data across the NHS system.

Linking VBP and a Sustainable NHS
A co-authored report establishing the link between value based procurement and delivering a sustainable NHS.

Introducing Our New Operating Model
A detailed look at the structural changes we’ve made to our organisational design, with the insourcing of six key Category Towers.

Product Innovation for Suppliers
An overview of how we are working with partners to enable suppliers to introduce new technology and innovation to the NHS.

Advancing Healthcare Programme
CEO, Andrew New, spoke to ITN Business about how NHS Supply Chain is helping to advance healthcare.
Helpful Information for Suppliers
A Net Zero Supply Chain and Suppliers
Suppliers are key to supporting sustainability targets. Keep up to date with the latest news and find out more about the five supplier requirements.
Value Based Procurement
The latest information and case studies highlighting how we are working the NHS to shift from cash releasing savings to value based procurement.
Supplier Portal
Information on how to access the supplier portal, an online system to support supplier management tasks.

