Innovative Footwear Clinically Proven to Reduce Knee Pain and Improve Joint Function
Over 20 million people in the UK, almost one third of the population, have a Musculoskeletal (MSK) condition such as arthritis or back pain.
People are living longer with complex MSK conditions and with over 15.3 million people in the UK expected to be over 65 years of age by 2030, there is in an ever-increasing demand on MSK services.
MSK conditions are more common in areas of greater poverty and may affect some ethnic groups more than others, how patients access, and experience care can also vary.
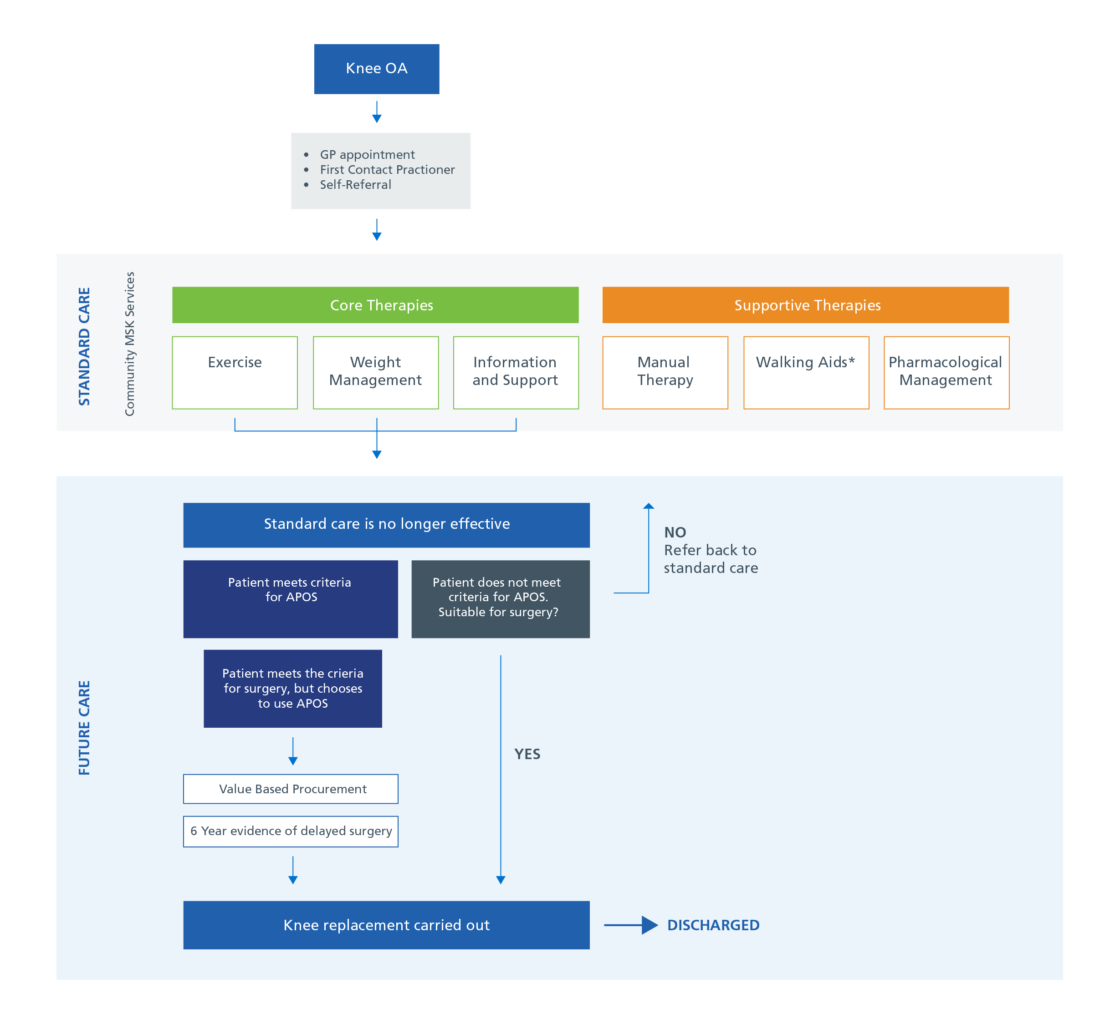
Currently, there is no standard treatment pathway for managing patients with knee osteoarthritis (OA). Patients are offered a variety of pharmacological and non-pharmacological options such as exercise, weight loss, pain relief and walking aids.
Once patients experience joint symptoms (pain, stiffness, and reduced function) that have a substantial impact on their quality of life or standard care treatment is no longer effective, they should be considered for surgery.
AposHealth® Solution Intervention
Apos® is targeted for those patients who would normally be referred to surgery and works on biomechanical and neuromuscular levels.
This is an innovative, non-invasive medical device (shoe) that is personalised to treat the cause of a patient’s pain, by retraining their gait (how they walk) to help aid better movement.
This National Institute for Health and Care Excellence (NICE) recommended medical device is clinically proven to relieve pain and improve function in the knees and lower back.
The proposed pathway illustration (below) shows how Apos® can be used before surgical intervention.
Two customised pods are attached to a bespoke shoe/boot device, which patients are required to wear for up to an hour a day, the pods are individually designed to meet patient needs.
Neuromuscular retraining
The pods’ convexity creates micro-instability that increases proprioception and re-educates neuromuscular pathways.
Biomechanical redistribution
Customised pod positioning changes the patient’s biomechanics, improves their gait, and directs forces away from unhealthy joint compartments.


Proposed Pathway Diagram
See our Downloads ▼ section to view a printable PDF version.
MedTech Funding Mandate
From April 2024 Apos® will be covered by NHS England’s MedTech Funding Mandate policy (MTFM). The MTFM aims to ensure patients and the NHS benefit from clinically effective and cost saving medical technologies faster and more equitably.
Technologies covered by the MTFM are typically funded by commissioners from their existing allocations. This is deliverable as the technologies supported are NICE recommended and meet the criteria to save costs and resources and make a return on investment within three years.
NHS Supply Chain will work alongside the Health Innovation Network (HINs) and APOSHealth® to clinically implement this product. To find out more information, please contact our Clinical Nurse Advisors.
Useful Links
-
NHS England Best Practice Solutions - Musculoskeletal
Find more information on NHS England's website.
-
APOSHealth®
Further research on this product can be found here.
-
NICE Recommendations
View the NICE recommendation here.
-
MedTech Funding Mandate
View information about the MedTech Funding Mandate and the products available through our supply route.
-
Clinical Nurse Advisors
Our Regional Clinical Nurse Advisors can help to improve the link between procurement and clinicians.
